STENT COATING SERVICES
 An Associate member of Thin Film Partners has developed proprietary coating methods and technology to uniformly and reproducibly coat coronary stents, peripheral stents, neurovascular stents, and coils, and similar prothesis. We can now provide coating services for both medical device manufacturers and academic institutions for a variety of applications.
An Associate member of Thin Film Partners has developed proprietary coating methods and technology to uniformly and reproducibly coat coronary stents, peripheral stents, neurovascular stents, and coils, and similar prothesis. We can now provide coating services for both medical device manufacturers and academic institutions for a variety of applications.
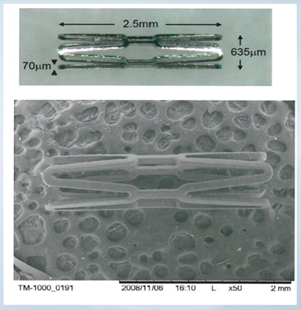
We will provide services ranging from coating formulation (drug, no drug, polymer, solvent carrier) to stent crimping and sterilization. Our coating experience is in processing both permanent (biostable) and biodegradable polymeric formulations on metallic and biodegradable stent substrates. We have coated a variety of stent sizes including small configurations for mouse implantation and drug testing, a “mouse stent” with a 0.635 mm diameter and an overall length of 2.5 mm; and large diameter peripheral devices with a 7.00 mm diameter and an overall length of 40
mm.
We are experienced in coating a variety of drug compositions/combination including Sirolimus (Rapamycin), Everolimus, Biolimus A9, Zotarolimus, Paclitaxel, Pimecrolimus, Trapidil, NSAIDs (Non-steroidal Anti-Inflammatory Drugs) and proprietary protein drugs. In addition, we provide formulated polymer compositions including, but not limited to the following: PLGA [Poly(DL-lactide-coglycolide)], PLA [Poly(DL-lactide) and Poly(L-lactide)], PCL [poly(e-caprolactone) and Poly (dL-lactide-co-e-caprolactone)], and fluorinated polymers. Thin Film Partners will support customers in all phases of their development. We have supported drug device development companies seeking to coat a variety of devices with their specific drugs. We provide coated components and coating technology to customers, saving the customer both process development time and eliminating the need to purchase custom specific and expensive tooling and process equipment.
Providing the following key services.

• Coat stents with polymer or polymer and drug
• Provide coating weights of polymer, drug, by use of 0.1 microgram balance
• Provide formulation guidance, including the selection of polymer, solvent, and drug to polymer ratio
• Capabilities include the following secondary services
o Inspection and sorting both bare metal stents (BMS) and
biodegradable stents
o Cleaning stents
o The application of primer(s)
o Providing stents
o Providing balloon catheters
o Crimping of stents onto catheters
o Packaging
o Sterilization
• Other unique customer services, as requested
Stent Coating Services
- Developed proprietary coating methods and technology to uniformly and reproducibly coat coronary and peripheral stents.
- Provides coating services to both device producers and academic institutions in all phases of their product development.
- Provides services ranging from coating formulation to stent crimping and sterilization.
- Provides coating formulations (polymers, drugs, solvent carrier system) for specific indications.
- Provides both permanent (biostable) and biodegradable polymers.
- Provides formulated drug compositions including, but not limited to
– Sirolimus (Rapamycin)
– Everolimus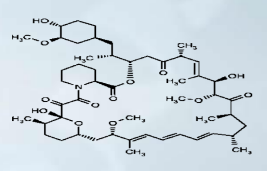
– Biolimus A9
– Zotarolimus
– Paclitaxel
– Pimecrolimus
– Trapidil
– NSAIDs (Non-steroidal Anti-inflammataory Drugs)
– Proprietary protein drugs - Provides formulated polymer compositions including, but not limited to
PLGA – Poly (DL-lactide-co-glycolide)
PLA – Poly (DL-lactide) and Poly (L-lactide)
PCL – Poly (e-caprolactone) and Poly (DL-lactide-co-e-caprolactone) - Provides coating services and methods for coating both metal and polymeric substrates.
- Provides coated components and coating technology to customers, thereby saving the customer/partner both (1) process development time and (2) the need to purchase custom specific and expensive tooling and process equipment
- Provides formulation guidance, including the selection of the polymer, the drug or no drug, and the solvent system.
- Will coat the devices with the selected polymer or polymer/drug composition(s).
- Provides coating weights of the coating, by use of the 0.1 microgram Mettler UMX Microbalance.
Has the capabilities to provide the following secondary services
- Incoming inspection and sorting of both bare metal stents (BMS) and biodegradable stents, devices
- Cleaning of devices
- Pretreatment of devices
- The application of primers
- Providing stents
- Providing balloon catheters
- Providing packaging
- Providing sterilization
- Providing other unique and customer specific services
Thin Film Partners Services
- Inspected and sorted CE approved bare metal stents (BMS)
- Cleaned the stents
- Applied primer to the stents
- Coated the stents with specified polymer/drug/solvent composition
- Inspected the coated stents
- Documented the polymer/drug coating weights
- Provided specific balloon catheters
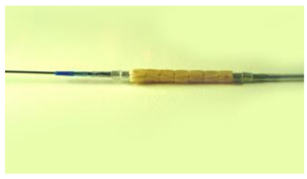
- Crimped coated stents onto catheters
- Packaged, double pouch, with desiccant and polymer/drug stabilizers
- Sterilized the devices
- Provided polymer certification
- Provided drug certification
- Provided sterilization documentation
- Shipped devices to locations specified by customer
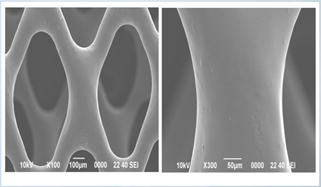
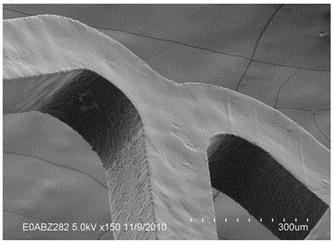
Thin Film Partners Coating Example Small Stent
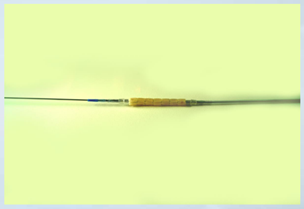
Thin Film Partners Coating Example Metal Coronary Stent 3.0mm in Diameter and 18.0mm long

Thin Film Partners Coating Example Biodegradable Coronary Stent 3.0mm in Diameter and 15.0mm long
Thin Film Partners Coating Example Biodegradable Peripheral Device 7.0mm in Diameter and 40.0mm long

Thin Film Partners Coating Example Biodegradable Peripheral Device