- Why is adhesion important for coatings?
- How do you achieve good adhesion for coatings?
- What is the adsorption mechanism for adhesion of coatings?
- What is Chemical Bonding for adhesion of coatings?
- What is Mechanical Interlocking for adhesion of coatings?
- What is surface contamination?
- What are the effects of contamination on the adhesion of a coating?
Why is adhesion important for coatings?
The durability and performance of coatings can depend on two basic properties. These are the cohesion and the adhesion of the coating.
Cohesion is the inner strength of a material and is associated with the strength of the molecular forces within the bulk material. Adhesion is the strength of the bonds forming between the coating and another surface.
Cohesive failure is usually in the coating film itself although it also could be within the substrate.
Adhesive failure can be a blister forming at the interface, lifting (delamination) of the coating, or any other situation that results from low adhesion at the interface.
Both cohesion and adhesion are required for a long-lasting, protective coating. Failures related to adhesion will determine the life of the coating on the product.
How do you achieve good adhesion for coatings?
Generally, technical coatings must exhibit good adhesion in order to be effective. However, there is no single theory that describes the property of adhesion.
But, there are several basic mechanisms for coatings that are known to define good adhesion. They are Adsorption, Chemical Bonding, and Mechanical Interlocking:
- Adsorption is where the molecules in the coating wet or flow freely over the substrate and make intimate contact with the substrate, forming interfacial (electrostatic) bonds with van-der-Waal forces.
- Chemical bonds are formed at the interface between the coating and the substrate.
- The coating film penetrates the roughness on the substrate surface. This results in mechanical interlocking once the coating dries.
All three of mechanisms do not have to occur to form good adhesion. Depending on the specific coating system, substrate, and application method, different mechanisms could work. However, good wetting or adsorption is normally required.
What is the adsorption mechanism for adhesion of coatings?
The theory of Adsorption states that good adhesion occurs from molecular contact between two materials and the surface forces that are developed. A bond develops from the adsorption of coating molecules on the substrate and the resulting attractive forces, usually designated as secondary (van der Waals) forces.
Electrostatic Mechanism
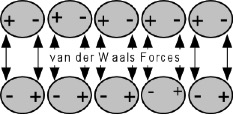
For these forces to develop, the respective surfaces must not be separated by more than five angstroms in distance. Therefore, the coating film must make intimate, molecular contact with the substrate surface.
The process of establishing continuous contact between a coating and the substrate surface is known as wetting.
Good wetting occurs when the coating flows into the valleys and crevices on the substrate surface. Poor wetting results when the coating bridges over the surface irregularities.
In order for good wetting to occur, the substrate must have a higher surface energy than the coating film. Many materials have a higher surface energy than the coatings being applied. Therefore, wetting is simple. However, if the substrate is contaminated with a lower surface energy material then wetting may be prevented.
Also, if the substrate is contaminated with particulates then a weak boundary layer could be formed that can easily fail cohesively and therefore weaken the coating system.
What is Chemical Bonding for adhesion of coatings?
Certain coatings are formulated with binders that have a functional group that can chemically bond to a compatible substrate. In these applications the formation of covalent chemical bonds occurs across the interface. These strong and durable bonds are generally the result of close contact or adsorption of the adhesive on the surface followed by a chemical reaction.
Coating systems containing reactive functional groups, such as hydroxyl or carbonyl, tend to adhere more tenaciously to substrates containing similar groups. Hydroxyl bonding is one of the reasons why epoxy and polyurethane base polymers are often used in in many formulations.
Perhaps the most widely employed example of chemical bonding in the coatings industry is with adhesion promoters or coupling agents. These multifunctional chemicals provide a “molecular bridge” between the substrate and the molecules in the coating film. One end of the adhesion promoter molecule has functionality that will react with the coating, and the other end has a functionality that will react with the substrate. A strong and durable bond forms as the coating cures.
Organosilane is an example of a widely used adhesion promoter. They are employed as additives in many coating formulations and as primers to promote adhesion, improve moisture resistance, and reduce the potential of corrosion at the interface.
What is Mechanical Interlocking for adhesion of coatings?
The surface of a solid material is never truly smooth. It normally consists of a maze of peaks and valleys. These are pores, holes, crevices and micro-voids on the substrate. According to the mechanical theory of adhesion, in order to function properly a coating must penetrate the irregularities on the surface, displace the trapped air at the interface, and lock-on mechanically to the substrate.
Mechanical Mechanism

One way that surface roughness aids in adhesion is by increasing the total contact area between the coating and the surface. If interfacial or intermolecular attraction is the basis for adhesion, increasing the actual area of contact will increase the total energy of surface interaction by a proportional amount.
Another benefit of mechanical interlocking is that a rough surface will provide a crack propagation barrier and stop the coating lifting from the substrate.
For a smooth interface, little energy dissipation may be required to separate the coating from the surface and a clean separation is possible. However, if there is surface roughness, then the interface between the adhering materials will act as “path breaks” between the separating coating and substrate. These excursions dissipate energy and increase the resulting strength of the bond.
Thus, there are many cases where the forces of adhesion and mechanical interlocking are working together at the substrate interface.
What is surface contamination?
According to Weldon (2009) surface preparation is a critical factor in obtaining good adhesion. Soils and other contaminants inhibit adhesion through though both chemical and physical pathways. By reducing the influence of intermolecular forces between the surface and the applied material, or by blocking sites that provide an opportunity for physical anchoring.
Also Schuman and Thames (2005) state that a variety of important coating properties such as hardness, scratch resistance, chip resistance and gloss are affected by the underlying surface’s hardness, smoothness and adhesion to the coating.
What are the effects of contamination on the adhesion of a coating?
Contamination that comes between a coating and the substrate surface can weaken the intermolecular electrostatic forces (van der Waal) and interfere with mechanical interlocking.
